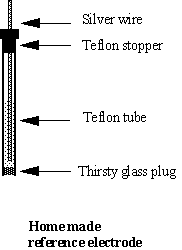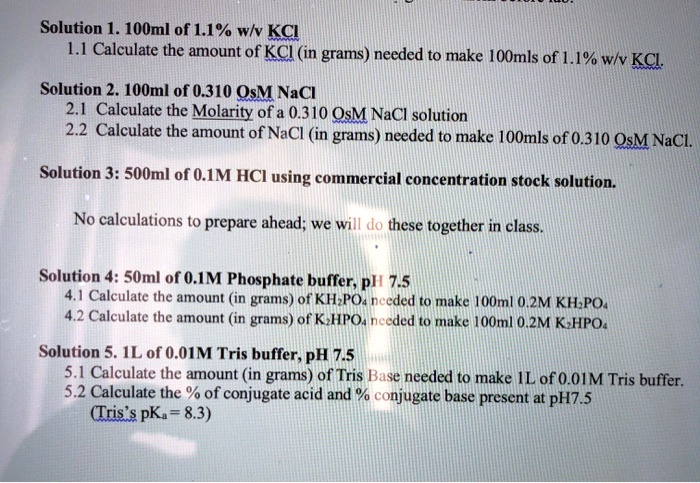
SOLVED: Solution 1. 1OOml of 1.1% wlv KCL L.1 Calculate the amount of KCI (in grams) needed to make IOmls of 1.1% wlv KCL Solution 2. 1OOml of 0.310 OsM NaCl 2.1
PDF) Simple procedures to prepare conductivity cell and pH solution & measure Conductivity AQC Solution

A solution is prepared by dissolving 4 g of NaOH to give 500 ml of it. Calculate the molality of the solution.