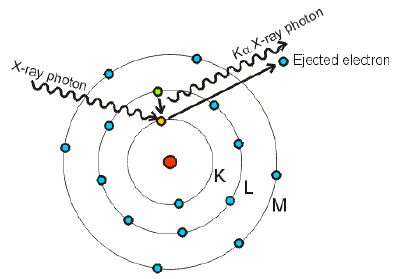
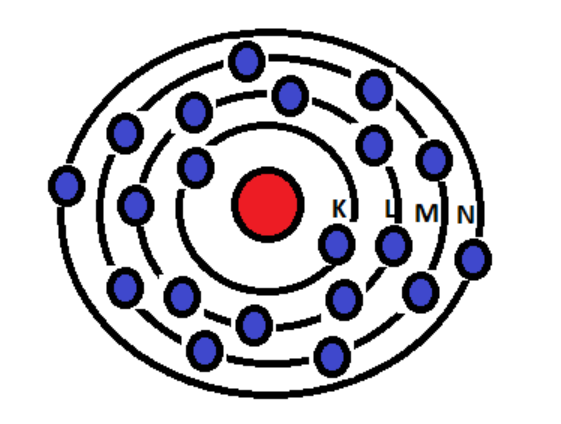
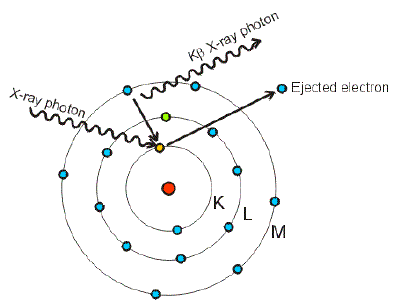
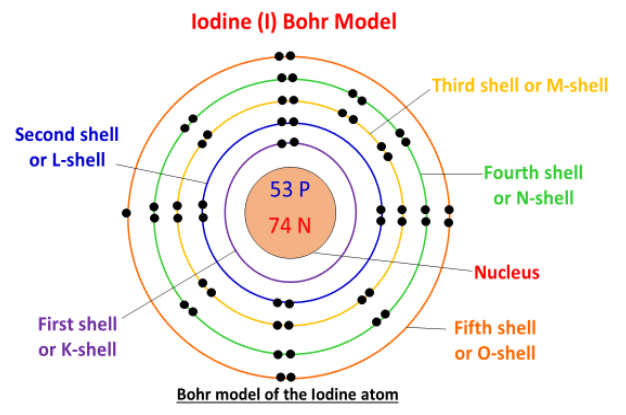
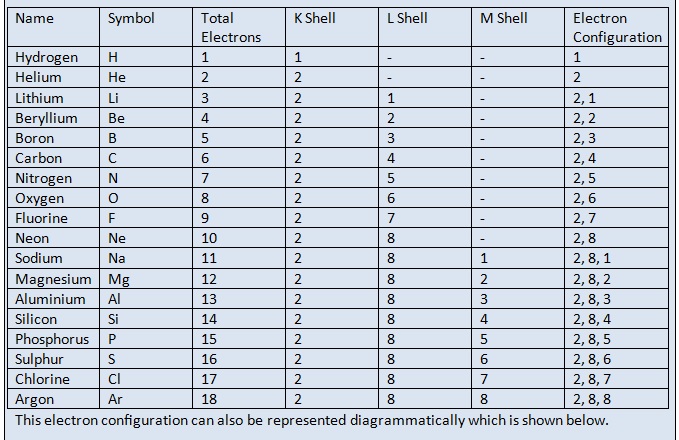
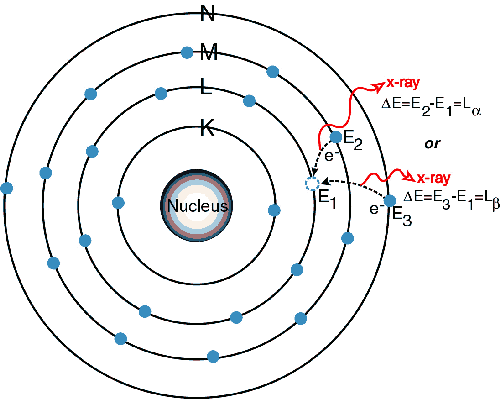
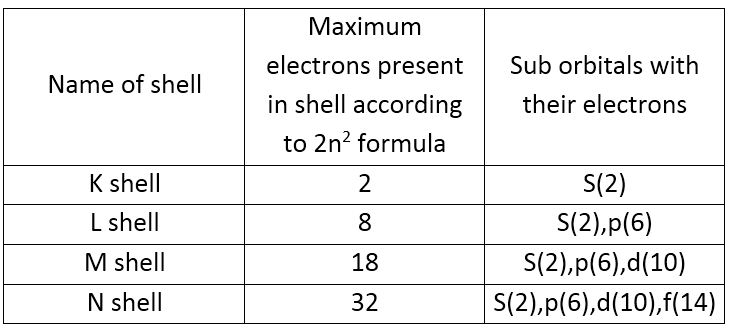
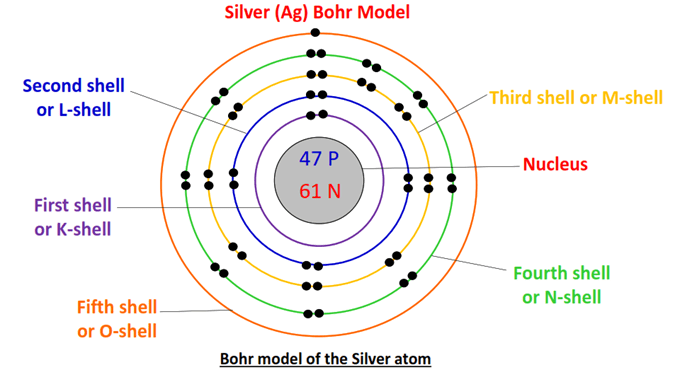
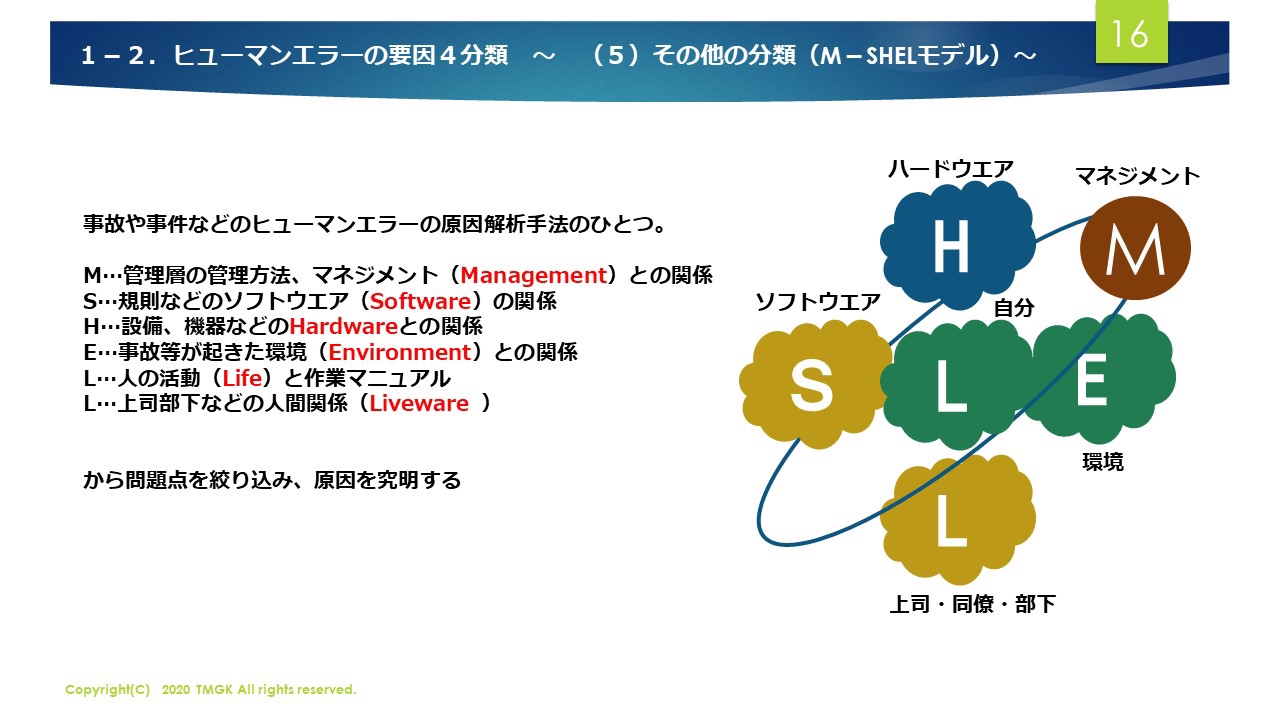
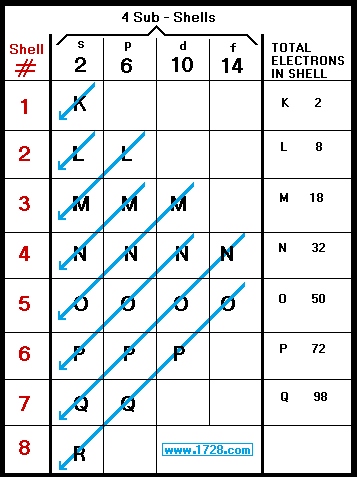
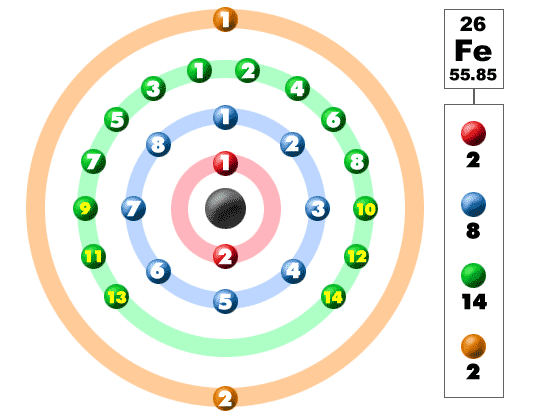
Electronic Configuration: Why M shell of Potassium has just 8 electrons instead of 9 electrons???? - YouTube

If K and Lshells of an atom are full and in Mshell there is only one electron then what would be the total number of electrons in the atom Name the element

What is the difference between Shell , Subshell and Orbital – Digital Kemistry - Best Online Free Chemistry Learning